Enhanced Biological Phosphorus Removal During Simultaneous Nitrification and Denitrification
Point and nonpoint source nutrient discharges to surface waters can lead to eutrophication and endanger aquatic life. After years of eutrophication, the Tampa Bay Estuary Program (TBEP) was successful in restoring Tampa Bay water quality by controlling the discharge of point sources, such as wastewater effluent (Morrison et al., 2011). According to the National Pollutant Discharge Elimination System (NPDES), treated effluent at Hillsborough County wastewater plants, including the Falkenburg Advanced Wastewater Treatment Plant (FAWTP), must contain no more than an annual average of 3 mg/L of nitrogen (as N) and 1 mg/L of phosphorus (as P) when it’s discharged to the surface water.
Biological and/or chemical processes can be used for removal of nitrogen and phosphorus from wastewater. Biological nitrogen removal uses ammonia-oxidizing microorganisms (AOM) and nitrite-oxidizing bacteria (NOB) to oxidize ammonia to nitrate under aerobic conditions. Subsequently, denitrifying microorganisms transform nitrate to nitrogen gas (N2) under anoxic conditions. Enhanced biological phosphorus removal (EBPR) uses polyphosphate-accumulating organisms (PAOs) to release phosphorus from bacterial cells under anaerobic conditions, and then take up phosphorus under anoxic or aerobic conditions, yielding a net removal of phosphorus from the aqueous solution.
Chemical techniques for phosphorus removal are based principally on the addition of coagulants, such as aluminum sulfate (alum), ferric chloride, or lime, all of which can result in the precipitation and subsequent sedimentation of a solid compound, such as AlPO4 (Tchobanoglous et al., 2014).
Biological treatment is usually preferred in Florida, where warm temperatures favor EBPR; however, biological treatment does not always meet the requirement for phosphorus removal, so it’s frequently accompanied by chemical addition to remove the remaining phosphorus.
Related Topics:
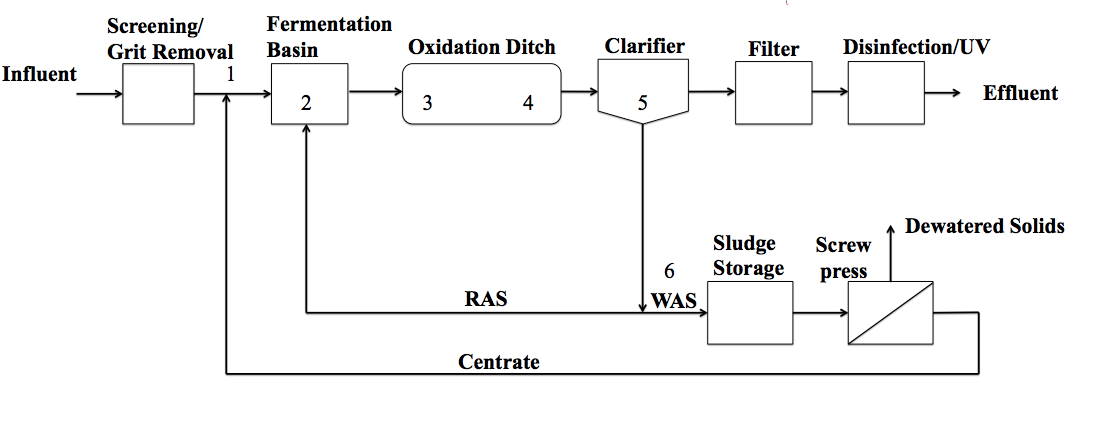
Different reactor configurations are used for implementing biological removal of nutrients. In many of these configurations, such as A2O or Bardenpho, separate basins or zones are required with different oxidation-reduction conditions and carbon sources in each basin to induce the required conditions. In contrast, an oxidation ditch (OD) is a configuration in which only one reactor is used instead of separate aerobic and anoxic reactors for biological nitrogen removal. The OD can be preceded by an anaerobic stage to initiate EBPR. Many wastewater treatment plants across the United States, including FAWTP, employ ODs. The OD is an economical and efficient design in Florida where land costs are:
- Relatively low in many regions
- Effluent carbon, nitrogen, and phosphorus limits are strict
- Large numbers of utilities have practical experience with this technology
Previous research has shown that simultaneous nitrification and denitrification (SND) occurs in ODs (Rittmann & Langeland, 1985; Daigger & Littleton, 2000; Sager, 2016). The SND is believed to occur by any of three possible mechanisms (Daigger and Littleton, 2000):
- Bioreactor macroenvironment. A single bioreactor, such as an OD, can contain both aerobic and anoxic microenvironments, supporting nitrification and denitrification, respectively.
- Floc microenvironment. A biological floc can contain a gradient of oxygen concentration, such that nitrification occurs near the aerated surface of the floc and denitrification occurs in the anoxic interior of the floc.
- S Novel microorganisms. Microorganisms that use denitrification by PAOs in a “previously unrecognized pathway” are able to remove nutrients from wastewater (e.g., denitrification by PAOs).
Assessing Influent
Average concentrations alkalinity, nitrogen species, and phosphate at the Falkenburg Advanced Wastewater Treatment Plant.
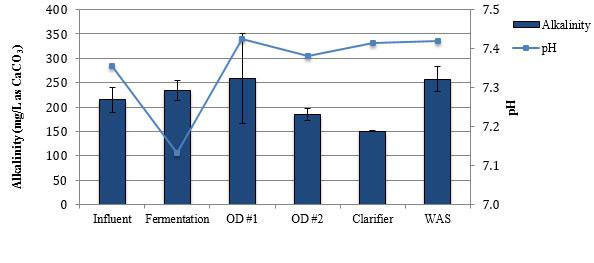
A number of prior studies have been carried out to assess nitrogen removal during SND (Rittmann & Langeland, 1985; Daigger & Littleton, 2000; Hao et al., 1997; Liu et al., 2010; Sager, 2016); however, the fate of phosphorus during SND is still not well understood. A few studies investigated phosphorus removal in SND systems at the bench scale (Zeng, 2003; Rout et al., 2007; Datta & Goel, 2010; Filipe & Daigger, 1999), pilot scale (Peng et al., 2007), and full scale (Littleton et al., 2003; Datta & Goel, 2010). Some researchers have reported the occurrence of EBPR during SND (Datta & Goel, 2010; Peng et al., 2007; Littleton et al., 2003; Zeng, 2003), but the removal mechanisms and expected removal efficiency are still not well understood. Further research is needed on the fate of phosphorus during SND to efficiently and reliably meet permit limits by employing an OD, and to minimize the cost of additional reagents for chemical precipitation.
The overall purpose of this study was to determine the fate of phosphorus during SND at FAWTP. More specifically, this article will:
- Assess treatment performance and SND occurrence at FAWTP
- Analyze phosphorus behavior and fate at FAWTP
- Compare the results of this study to previously published results
Excerpted from the January 2019 issue of Florida Water Resources Journal