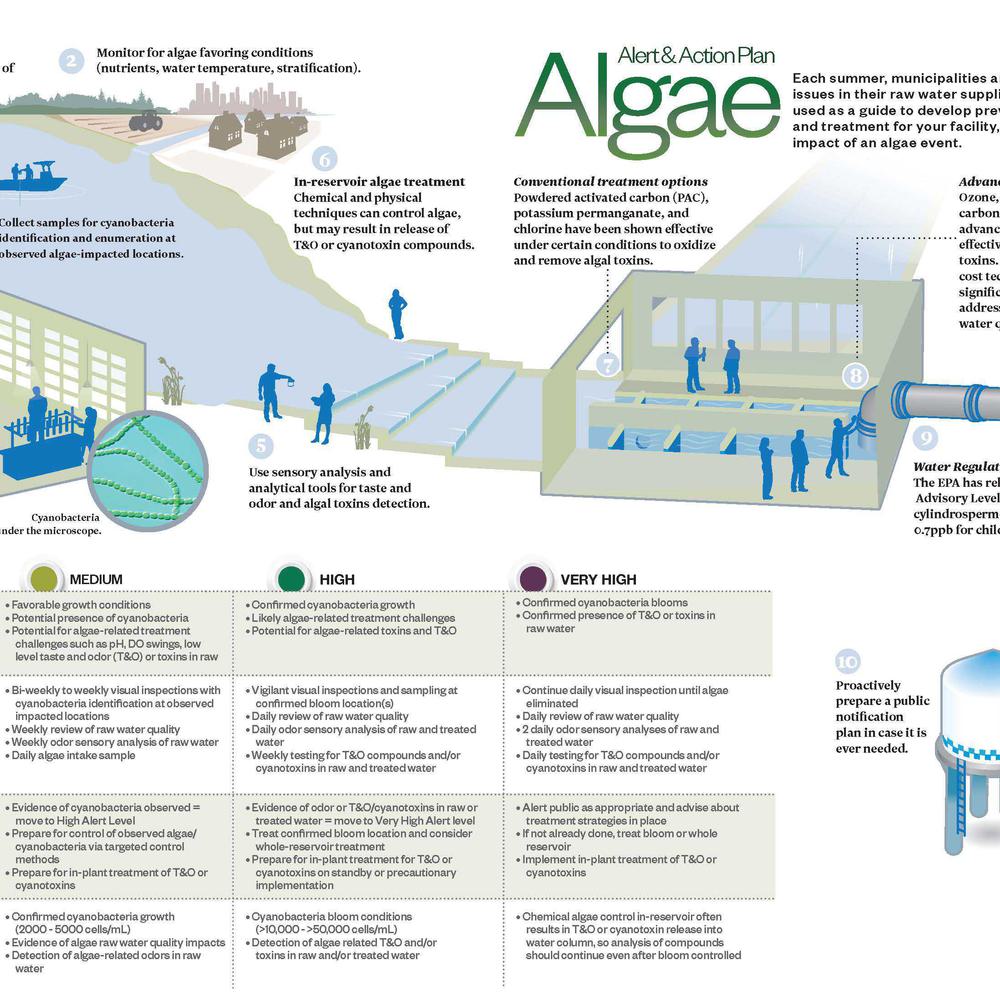
Practical Application of Lead Service Line Scale Analysis for Optimizing Corrosion Control
A major water utility performed laboratory scale analysis of harvested lead service lines (LSLs) as part of an initiative to optimize corrosion control treatment (CCT) with a voluntary Action Level goal of 5 ppb for lead in drinking water. The presentation will describe how the scale analysis results were used to inform the decision-making process for optimizing CCT and will include a comparison of the results to prior studies on LSL scale analysis.
Scale analysis can be utilized as a practical tool to understand mechanisms of corrosion control for LSLs. The type of scale formation is influenced by the water quality conditions and the type of CCT. The characteristics of the scale layer and the water quality determine lead release and lead solubility, and understanding the scale characteristics is an important aspect of optimizing CCT and evaluating future treatment changes. Characterizing the scale layer is an important step towards promoting scale stability and avoiding unintended impacts of water quality or treatment changes on existing scale layers.
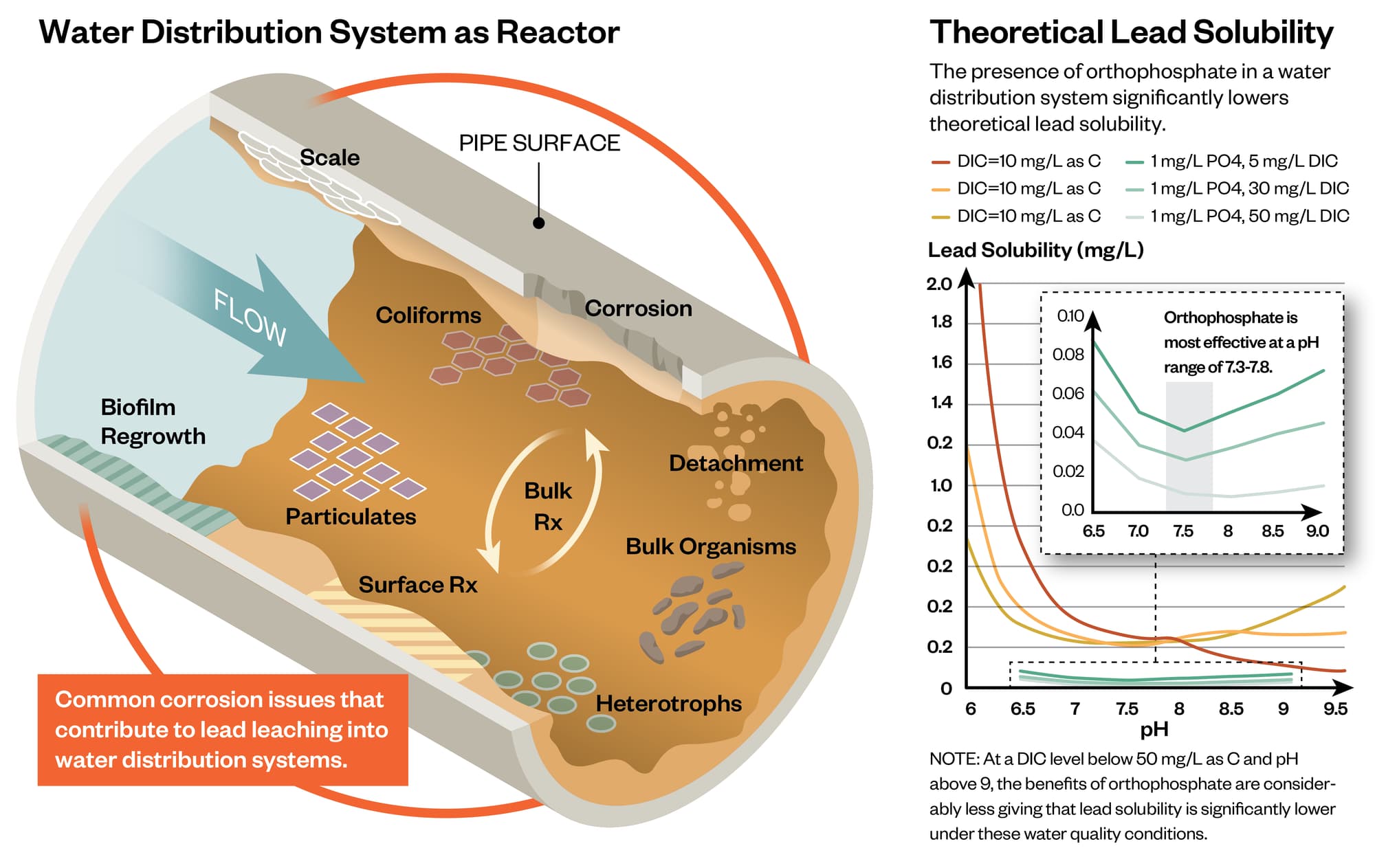
Two distinct scale layers were observed on each LSL sample. In general, the layer at the pipe wall was a lead carbonate scale, and the layer adjacent to the water contained amorphous deposits of aluminum, phosphorus, and iron, with small amounts of calcium and silica. The layer at the water interface contained a lower percentage of lead by weight compared to the layer on the pipe wall. LSL scale characteristics varied among sites, and the visual properties of one sample differed from other pipes in the same system.
The utility has added a blended phosphate corrosion inhibitor since the 1990’s. The LSL scales contained phosphorus, but crystalline lead-phosphate solids were not detected. Phosphorus present on the pipe wall may have reacted with aluminum or calcium in the bulk water rather than with lead. The presence of lead-carbonate scale and the apparent absence of crystalline lead-phosphate solids differs from the conventional theory and solubility models for phosphate corrosion inhibitors.
Aluminum was the primary non-lead constituent in each LSL sample, and the scale likely contains a combination of aluminum hydroxide, aluminosilicate, and aluminum phosphate solids. Based on reports in the literature, these aluminum deposits may form a “diffusion barrier” to reduce lead release from the pipe wall into drinking water.
The existing LSL scale layer must be considered in planning corrosion control pilot tests and interpreting results of corrosion studies. The impacts of CCT and other treatment changes should be evaluated relative to actual scale layers present in the distribution system by utilizing harvested LSLs for pilot studies. The utility is planning a pilot scale pipe loop study and will monitor changes in LSL scale speciation due to alternate corrosion inhibitor types and doses.